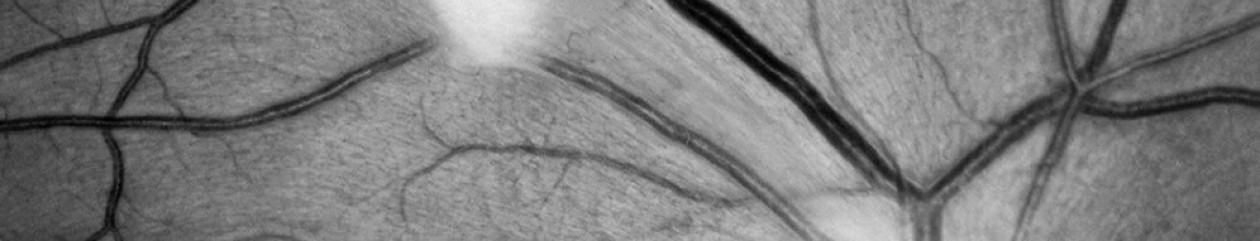
First time viewers of ophthalmic images frequently make the observation that the photos look like something from outer space. Especially when reviewing the round orange retinal photos with their eye doctor, patients often comment, “That looks like the planet Mars.”

Every time it happens I get a chuckle out of it. As if we all truly know what the planet Mars really looks like! But to most people, images of the inside of an eye are foreign and amazing. And there does seem to be a little science fiction aspect to both the appearance of the eye when viewed at high magnification, as well as the technology used to capture these amazing images. There are however, several space analogies that really seem to ring true. Among eye-care professionals, the eyeball is routinely referred to as the “globe”.

Many clinical findings are named by their appearance rather than an underlying cause, and several conditions have names derived from their similarity in appearance to objects in space: asteroid hyalosis, macular star, star folds, starry sky, astrocytoma, stellate pattern, etc.

In fact, there are enough conditions like this, that I’ve been able to compile them into the Ophthalmic Jeopardy category: Celestial Bodies.

Like images from space, there does seem to be an element of wonder and mystery when we peer inside the globe, so in some ways the analogy makes sense.
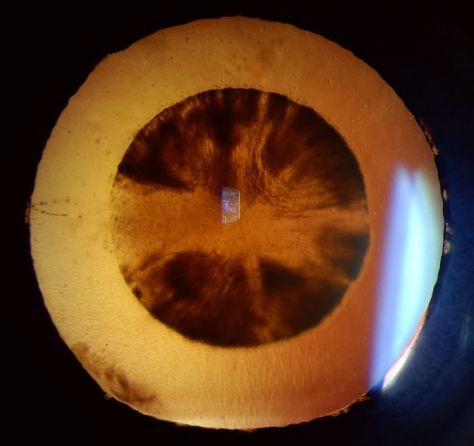
Many ophthalmic images seem reminiscent of photographs from NASA. Or they may stir our imagination or perception of how objects in space might appear.

There are other connections as well. Some of the photographic techniques used by both astronomers and ophthalmic photographers are actually similar. IR capture, interferometry and stereo imaging are common techniques in both fields. The principles of rotational stereo imaging can be applied to both subjects. Filters or lasers of different wavelengths are commonly used to enhance visibility of certain features in both subject types.
Most of these analogies between the eye and outer space, are loose associations rather than a direct connection. There is however, at least one eye condition that can be directly associated with a celestial body. Solar retinopathy is a type of photic injury to the retina that is the result of staring at the sun. This condition typically occurs in patients with psychiatric disorders or under the influence of hallucinogenic drugs.

Some scholars believe that early astronomers, especially Gallileo, went blind as the result of solar retinopathy from viewing the sun through a telescope. It’s important to note that this condition can also occur from viewing a solar eclipse without protective eyewear. The upcoming solar eclipse visible in the U.S. on August 21, may cause a spike in cases of solar retinopathy presenting to emergency rooms and eye clinics. The American Academy of Ophthalmology offers some tips for safe viewing of the eclipse.
In recent years, another connection between outer space and vision has been discovered. It turns out that space travel can have some damaging effects on the human eye. Long-term exposure to microgravity can lead to a hyperopic shift in vision from flattening of the globe. This condition is believed to be related to increased intracranial pressure and is sometimes associated with optic disc edema, cotton wool spots and choroidal folds. Optical coherence tomography (OCT) is used to document changes in thickness of the retinal nerve fiber layer of astronauts before, during, and after space flight.

I took this OCT selfie a few years back when we had a scientist from NASA visiting our clinic while exploring the possibility of putting an OCT on the International Space Station. She wanted to see the Heidelberg Spectralis in clinical use. After demonstrating on several patients, the scientist asked me if I thought it were possible for someone to take an OCT image of themselves. I pivoted the monitor, control panel, and footswitch around so I could operate the OCT from the patient chair and then captured some images of my own retina. I was showing off a little and smugly cautioned the NASA doctor that this was a difficult feat that only an experienced ophthalmic imager could perform. After all, I’ve been doing this for over thirty years. She paused for a moment and then said, “With all due respect, astronauts are some of the smartest and most talented people on earth. They shouldn’t have any difficulty performing OCTs on themselves after a some brief training.” Suddenly I didn’t feel so smug.

A year or so later, the Spectralis arrived at the International Space Station and it looks like she was right. I heard from some colleagues at Heidelberg that the astronauts were given less than 30 minutes of training on the instrument and mastered it quickly!

It’s pretty cool knowing that astronauts are performing ophthalmic imaging on the International Space Station. I wonder if they ever see any resemblance between the eye and celestial bodies?

Disclosure: I have no financial or proprietary interest in the Heidelberg Spectralis.
Here are some links on the condition that’s effecting the vision of astronauts and the use of diagnostic imaging on the space station:
http://www.vision-research.eu/index.php?id=858
https://www.theatlantic.com/science/archive/2017/01/seeing-in-space/513650/
http://www.utsouthwestern.edu/newsroom/news-releases/year-2017/jan/vision-levine.html