In the past month or so we’ve increased our testing volume after limiting patient visits during a government mandated stay-at-home order during the COVID 19 pandemic. As we’ve ramped back up, we’ve changed our procedures to limit the risk of transmitting the virus. We’ve instituted stringent cleaning protocols, and installed breath shields on some instruments. All clinical personnel now wear PPE (masks, goggles, gloves) and all patients are required to wear masks while in the clinic. We’ve adapted to this “new normal” pretty quickly and our testing volume is almost back to pre-pandemic levels.
Another part of our new normal is a novel clinical finding that seems to be related to the COVID 19 pandemic. Prior to the pandemic, I hadn’t observed this finding. As our numbers increased, I started noticing artifacts that I couldn’t immediately identify in IR fundus images using the Spectralis OCT. I’m wondering how many others in the field have noticed the same thing.

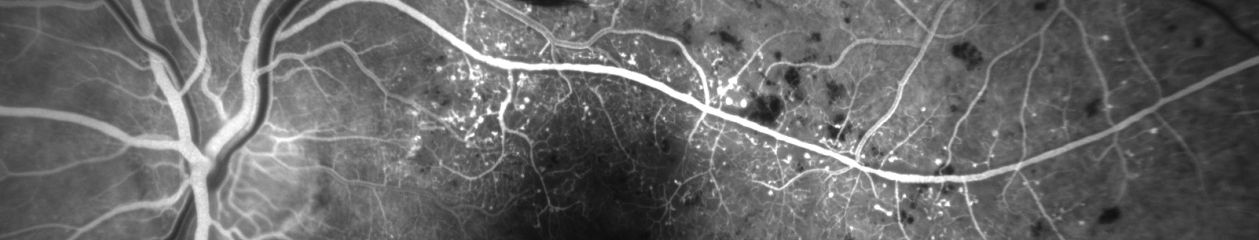
This patient came in two weeks ago. Here you see what appears to be vitreous hemorrhage or debris causing a dark shadow that obscures the view in the IR image. I really struggled to get good images. The OCT was adequate, but the accompanying IR fundus image was poor. Those of you that use the Spectralis know that any structure or pathology that is out of focus will typically appear dark because of the confocal pinhole that blocks scattered light from reaching the sensor. So it’s not uncommon to have a compromised view like that if there are media opacities such as dense cataracts, corneal opacities or vitreous pathology, so I didn’t think much of it at first.

But then just two patients later I saw a similar artifact. This time it changed in appearance while I was imaging and got progressively worse. I had the patient sit back and re-positioned.

That seemed to improve the view, but within seconds it deteriorated again and got worse. It almost looked as if I were watching a vitreous hemorrhage occur live. I had the patient sit back yet again, waited several seconds, and resumed imaging. The artifact disappeared again!

Finally, I looked around the device at the patient’s face to see if there was anything I was missing. And then I noticed it, the patient had an ill-fitting mask and was fogging the lens with her breath! The position of the mask forced her breath directly onto the lens surface of the Spectralis. Because of the confocal nature of the Spectralis, the pinhole aperture causes the artifact to appear dark rather than just a simple fogging seen with a slit lamp, fundus camera, or any other non-confocal device.

I asked a few of my astute imaging colleagues about this and a few had seen it but were initially stumped as well. Collectively we now believe we are seeing a new artifact related to patients wearing masks. One of my colleagues thought it was more prominent with the SPECTRALIS 102 degree wide angle lens. That may be because of the different working distance from the patient (closer).

Image courtesy Gary Miller, CRA, OCT-C, FOPS
Looking back I realized that I had struggled with many patients during the peak of the lockdown. We were only seeing urgent/emergent patients at that time and were not routinely dilating patients in the interest of efficiency. So I assumed the darker fundus images were related to working with smaller pupils. It dramatically effects the confocal fundus image, but not always the OCT image because that component of the device is not confocal. The fogging seems to be more common with certain mask types or on days with high humidity.
Now when it occurs, I often just ask the patient to sit back for a few seconds until the fog clears clears and then resume imaging. Or we sometimes tape the top of the mask to the patient’s nose and cheeks to direct their breath down or to the side.

Another trick is to change the OCT working distance (XL setting) as if you were trying to capture a longer eye. That effectively changes the angle between the patient’s mask and the front element.
My colleague Gary Miller and I have been unsuccessfully trying to come up with a catchy name for this finding. Here are a few of our attempts, but they’re all pretty lame.
Corona Fog
Pandemic haze
Mask mist
COVID condensation
Masquerade artifact
If you have a good one, put it in the comments section.
Here are the current recommendations from Heidelberg on cleaning optical surfaces in their devices. These recommendations seem pretty universal and would likely be similar to those from other manufacturers as well.

Update: A number of people have asked for a document to share whith coworkers. I created a quick pdf adaptation that can be found here.
Tim Bennett, CRA, OCT-C, FOPS






















 The footprint of the Eidon is fairly compact, but the instrument is taller than most fundus cameras. The device is operated via touch screen tablet and has both automatic and manual controls. The Eidon has a fixed 60 field of view, but is capable of capturing several fields and creating montage images. It features a 14 megapixel sensor to capture color, red free, and infrared images. The red free photos are extracted from the color image rather than through a separate exposure with a blue-green light source.
The footprint of the Eidon is fairly compact, but the instrument is taller than most fundus cameras. The device is operated via touch screen tablet and has both automatic and manual controls. The Eidon has a fixed 60 field of view, but is capable of capturing several fields and creating montage images. It features a 14 megapixel sensor to capture color, red free, and infrared images. The red free photos are extracted from the color image rather than through a separate exposure with a blue-green light source.



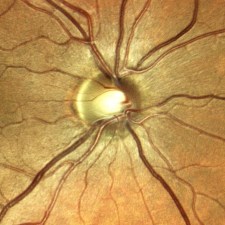 One of the features touted by the manufacturer is that it prevents “optic disc bleaching” seen with some fundus cameras. It does hold detail in optic disc photos, but the flip side to this is that the rim of the nerve can appear abnormally dark or gray, making it difficult to document pallor. Disc bleaching shouldn’t be a problem in fundus cameras that are
One of the features touted by the manufacturer is that it prevents “optic disc bleaching” seen with some fundus cameras. It does hold detail in optic disc photos, but the flip side to this is that the rim of the nerve can appear abnormally dark or gray, making it difficult to document pallor. Disc bleaching shouldn’t be a problem in fundus cameras that are 
























